| Product No. | CH024 |
|---|---|
| CAS Reg. No. | not available |
| Alternate CAS Reg. No. | 112811-59-3 (unlabelled compound) |
| Offer | 25 mg, 50 mg |
not available
- Documentation
- Details
Chemical name
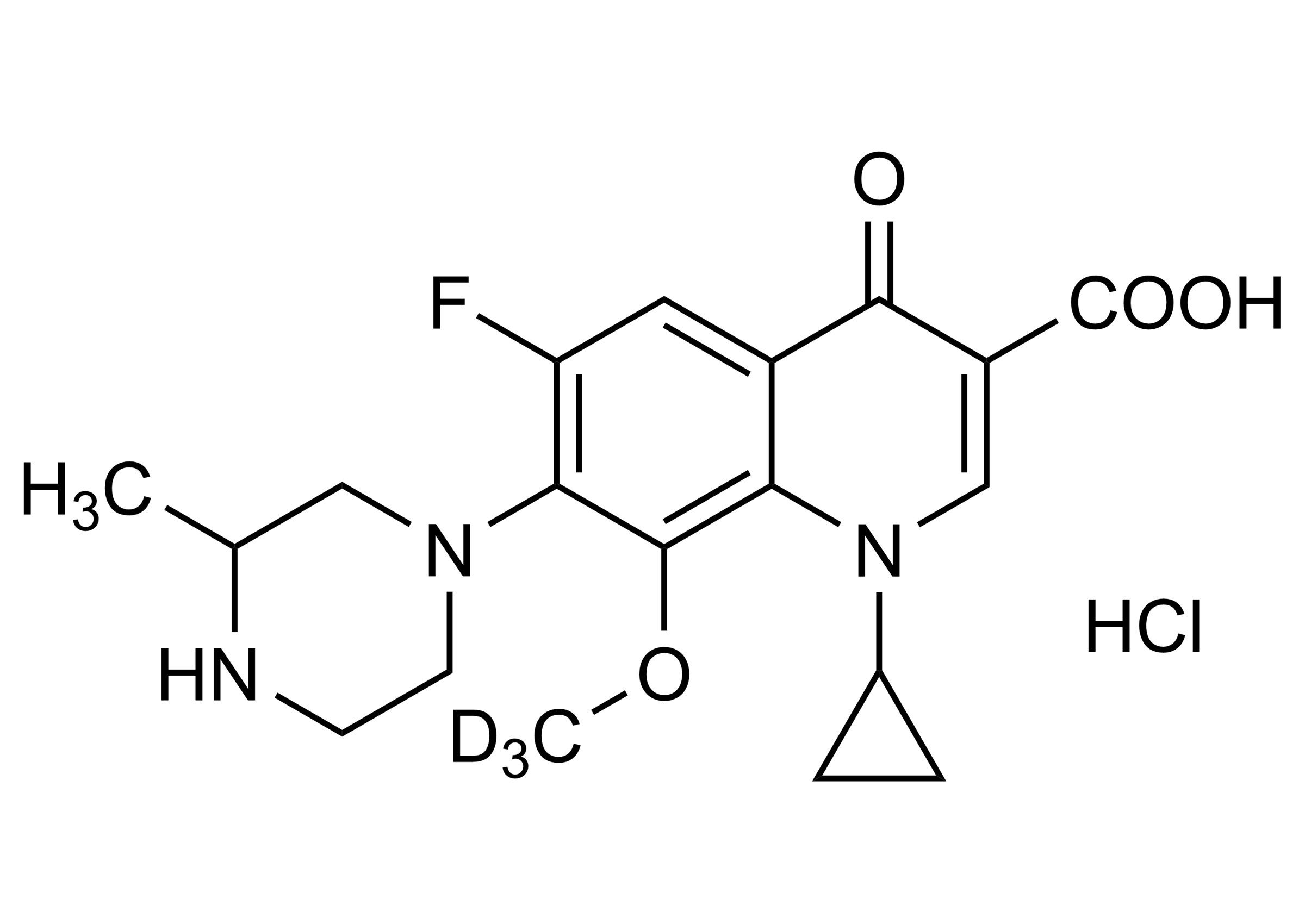
1-Cyclopropyl-6-fluoro-4-oxo-8-methoxy-D3-7-(3-methylpiperazin-1-yl)-1,4-dihydro-quinoline-3-carboxylic acid hydrochloride
Description
Gatifloxacin-D3 hydrochloride is a deuterated fluoroquinolone reference standard produced and distributed by WITEGA Laboratorien Berlin-Adlershof GmbH. Use it as an LC-MS/MS calibration solution in targeted assays. It also supports multi-residue method development standard workflows in food, environmental, and clinical matrices. This reference standard helps you achieve confident quantification and reliable identification from sample to report.
Optimized for LC-MS/MS and GC-MS, the material enables traceability across batches and instruments. Therefore, you can verify recovery, evaluate matrix effects, and check selectivity during method validation. Moreover, it streamlines confirmatory analysis by providing a stable labeled analog that mirrors the native analyte.
- Quantification: establish linear calibration and dynamic range with robust response factors.
- Traceability: link results to documented batch data and a detailed certificate of analysis.
- Calibration: prepare working standards and matrix-matched levels with predictable performance.
- Method validation: assess accuracy, precision, LOQ, and measurement uncertainty.
- Confirmatory analysis: verify transitions and ion ratios under regulated criteria.
This reference standard from WITEGA Laboratorien Berlin-Adlershof GmbH fits diverse settings. Regulated laboratories rely on it for routine monitoring. Pharmaceutical research teams use it in metabolism studies and stability assessments. Residue control labs add it to quality control sequences to track drift and instrument health. Additionally, it supports multi-residue screening where consistent labeling improves peak confirmation.
- Intended use: LC-MS/MS or GC-MS development, system suitability, and ongoing QC.
- Typical matrices: plasma, urine, tissue, food, feed, and environmental extracts.
- Documentation: batch-specific CoA ensures transparency and reproducibility.
- Handling: follow your lab protocols for storage, dilution, and contamination control.
Choose Gatifloxacin-D3 hydrochloride when you need a traceable reference standard. It brings clarity to calibration, strengthens method validation, and enhances confirmatory analysis. Furthermore, partnering with WITEGA Laboratorien Berlin-Adlershof GmbH ensures dependable supply, technical support, and consistent quality.
Safety Data Sheet
You can download your Safety Data Sheet for CH024
For other languages please contact us: witega@witega.de
Additional information
| Chemical name | 1-Cyclopropyl-6-fluoro-4-oxo-8-methoxy-D3-7-(3-methylpiperazin-1-yl)-1,4-dihydro-quinoline-3-carboxylic acid hydrochloride |
|---|---|
| Molecular Formula | C19H19D3FN3O4 x HCl |
| Molecular Weight | 414.87 g/mol |
| Isotopic purity | >99.0 atom% D (1H NMR) |
| HPLC purity | > 99.0 % |
| Overall purity | > 99.0 % (HPLC) |
| Product Format | Neat |
| Delivery time | In stock |
| shelf life | 24 months |
| Storage | refrigerator, 2-8°C |
| Country of Origin | Germany |
| Product No. | CH024 |
| CAS Reg. No. | not available |
| Alternate CAS Reg. No. | 112811-59-3 (unlabelled compound) |
| Offer | 25 mg, 50 mg |